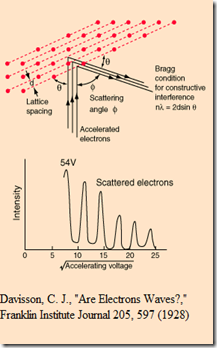
In 1924 Louis de Broglie proposed that all moving particles exhibit a degree of wave-like behavior then Thompson, Davidson and Germer have conducted experiments with fast moving electrons and found that moving particles can not go in straight line but make oscillation in their path. The electrons are accelerated by applying high voltages between the anode and cathode. But in an atom there is no force which can consistently accelerate its electrons and create “standing matter wave of electrons”.
Putting wave-particle duality on a firm experimental footing, it represented a major step forward in the development of quantum mechanics
sources :
http://hyperphysics.phy-astr.gsu.edu/hbase/quantum/davger2.html#c1



No comments:
Post a Comment